Buying and Using Silver Nitrate
Welcome to our comprehensive FAQ guide on buying and using silver nitrate. Whether you're a seasoned professional or a curious beginner, this guide aims to answer all your questions and provide valuable insights into this versatile compound.
1. What is Silver Nitrate?
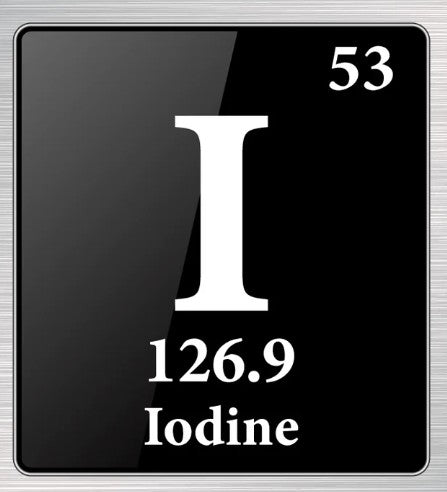
Silver nitrate is an inorganic compound with the chemical formula AgNO3. It is a versatile substance used in various fields, including medicine, photography, and industrial applications. Known for its antiseptic and staining properties, silver nitrate is a crucial chemical in many scientific and practical processes.
2. What Are the Common Uses of Silver Nitrate?
- Medical Applications: Used as an antiseptic and in the treatment of wounds.
- Photography: Historical use in developing photographic films.
- Industrial Uses: Involved in mirror production, chemical synthesis, and as a catalyst.
- Education and Research: Used in chemical reactions and experiments to demonstrate various principles.
- DIY Projects: Popular among hobbyists for staining and creating chemical reactions.
3. Where Can I Buy Silver Nitrate?
Silver nitrate can be purchased from:
- Chemical Supply Companies: Specialized suppliers offer various grades and quantities.
- Online Retailers: Websites like Amazon, eBay, and specialized chemical stores.
- Pharmacies: Some pharmacies may carry silver nitrate, particularly for medical use.
- Educational Suppliers: Companies that supply educational institutions often carry silver nitrate for laboratory use.
4. What Should I Consider When Buying Silver Nitrate?
- Purity: Ensure the silver nitrate is of the required purity for your intended use.
- Grade: Different grades (e.g., reagent grade, pharmaceutical grade) are suited for different applications.
- Supplier Reputation: Buy from reputable suppliers to ensure quality and authenticity.
- Quantity: Purchase the appropriate amount based on your needs to avoid excess storage.
5. How Should I Store Silver Nitrate?
- Temperature: Store in a cool, dry place away from direct sunlight.
- Container: Keep it in a tightly sealed, dark glass container to prevent light exposure.
- Labeling: Clearly label the container with the chemical name and hazards.
- Safety: Store away from incompatible substances and out of reach of children and pets.
6. What Safety Precautions Should I Take When Handling Silver Nitrate?
- Personal Protective Equipment (PPE): Wear gloves, safety goggles, and a lab coat.
- Ventilation: Use in a well-ventilated area to avoid inhaling fumes.
- Skin Contact: Avoid skin contact as it can cause staining and irritation.
- Emergency Procedures: Know the first aid measures for exposure or ingestion.
7. What Are the Environmental Concerns with Silver Nitrate?
Silver nitrate is toxic to aquatic life and can have long-lasting effects on aquatic environments. Proper disposal is essential:
- Disposal Regulations: Follow local regulations for hazardous waste disposal.
- Spill Management: Clean up spills immediately using appropriate methods and dispose of waste properly.
8. Can I Make Silver Nitrate Solutions at Home?
Yes, you can make silver nitrate solutions by dissolving the solid compound in distilled water. Ensure accurate measurements for the desired concentration and follow safety guidelines:
- Measuring: Use precise scales to measure the amount of silver nitrate.
- Mixing: Dissolve the silver nitrate in distilled water slowly while stirring.
- Storage: Store the solution in a labeled, dark glass bottle.
9. What Are Some Common Myths About Silver Nitrate?
Myth: Silver nitrate is only useful in photography.
- Reality: It has diverse applications in medicine, industry, and education.
Myth: It’s safe to handle without precautions.
- Reality: Proper safety measures are essential to prevent harm.
Myth: It can be disposed of down the drain.
- Reality: Proper disposal is necessary to prevent environmental damage.
10. Where Can I Find More Information and Resources?
- Books: Look for chemistry and industrial chemistry textbooks.
- Online Courses: Enroll in courses that cover inorganic chemistry and laboratory safety.
- Professional Organizations: Organizations like the American Chemical Society (ACS) provide resources and guidelines.
- Research Papers: Academic journals and papers can offer detailed information on specific uses and studies involving silver nitrate.
Understanding the properties, uses, and safety measures of silver nitrate is crucial for anyone looking to buy and use this compound. By following the guidelines and information provided in this FAQ, you can ensure safe and effective use of silver nitrate in your projects and research. If you have any more questions or need further assistance, feel free to reach out or leave a comment below.